Qualitative test for COVID-19 total Ab
The FREND™ COVID-19 total Ab is a fluorescence immunoassay (FIA) which can be used to aid in identifying individuals with an adaptive immune response to SARS-CoV-2 using human plasma. It shows qualitative result by using SARS-CoV-2 Nucleocapsid fluorescent beads to detect total antibody (IgG and IgM).
Key Benefits of FREND™ COVID-19 total Ab
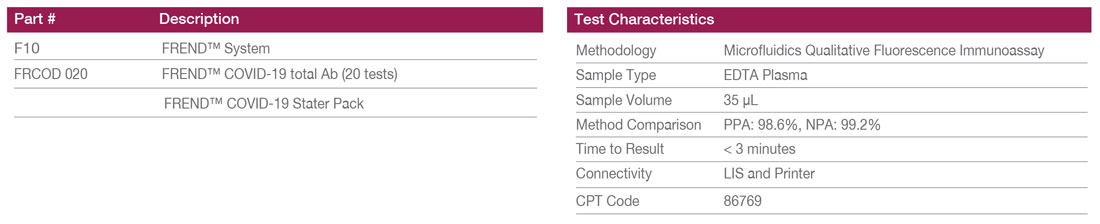
FREND™ System
Easy & Fast Operation
The results can be obtained in less than 3 minutes with only 35 µl of sample volume. The FREND™ System offers an intuitive and user-friendly interface. System QC and software updates are just a click away. Other tests available on the FREND™ System include: tPSA, TSH, FT4, Testosterone, and Vitamin D.
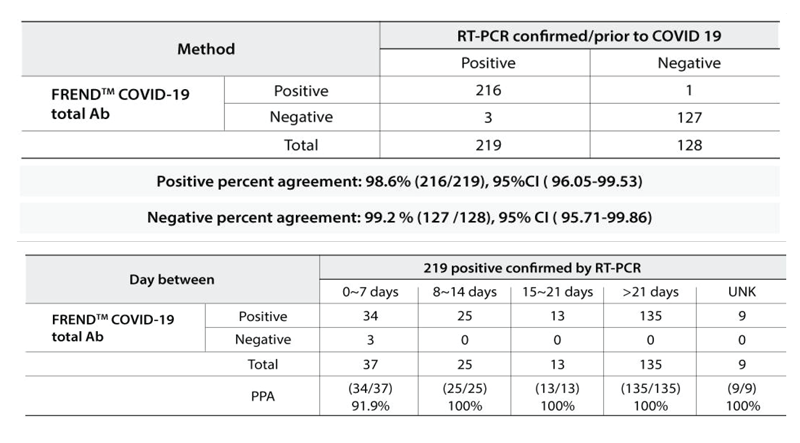
Clinical Performance Evaluation
The results of the total 347 clinical samples (219 RT-PCR Confirmed Positive SARS-CoV-2 and 128 negative, pre-COVID-19) are presented in the table below.
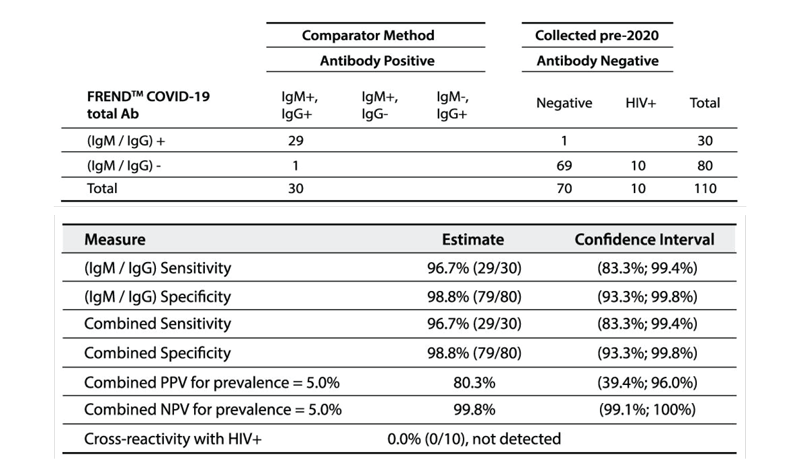
Independent Clinical Agreement Validation
The FREND™ COVID-19 total Ab from NanoEntek was tested on August 19, 2020 at the Frederick National Laboratory for Cancer Research (FNLCR) sponsored by the National Cancer Institute (NCI).